Interest in cannabidiol – better known as CBD – has recently exploded in America, with an estimated 1 in 7 adults currently using a CBD-based product. Brenan 2019 This is big business, with a projected commercial market of $80 billion by 2030 and $20 million in research funding in 2019. Franck 2019 Medgadget 2019 Of the nearly 45 million Americans using CBD, 62% report using CBD to treat a medical condition, e.g. pain, anxiety, and depression most commonly. Corroon 2018 Given its popularity, I thought I would dig into the current research on CBD Oil to see what the science says and if all the hype is warranted.
Key Points:
- Cannabidiol (CBD) is made from the flowers and leaves of one of the Cannabis species, Cannabis sativa, Cannabis indica, or Cannabis ruderalis. Varieties of these species (e.g., hemp and marijuana) are further categorized by their concentration of THC, the biologically active substance associated with the “high” from marijuana ingestion. It is currently legal to make and distribute hemp-derived products including CBD oil in all states, including interstate commerce. However, it is illegal to make and distribute marijuana-derived products across state lines, although a number of states have their own regulations for legalized marijuana use.
- The CBD industry is booming, with an estimated market of $80 billion by 2030. Unfortunately, there are significant concerns with the quality of products in the market and safety in general. For example, when ~80 different commercially available CBD products were tested, only 31% had their CBD concentration labeled accurately.Bonn-Miller 2017 Over half of all tested samples found additional chemicals in the product that were not on the label. Adverse reactions to CBD are also seen in about ⅓ of subjects including diarrhea and liver injury.VanDolah 2019
- The existing evidence on CBD is relatively good for its use in reducing seizure frequency in individuals with drug-resistant seizures. It also may be useful for reducing anxiety prior to public speaking. However, the existing data suggests it is not effective for pain, psychiatric conditions, Parkinson’s disease, insomnia, or exercise recovery, among others.
What is CBD Oil?
In biology, taxonomy is the branch of science that identifies, describes, names, and ultimately classifies different organisms. The taxonomy of CBD starts with Cannabis, a genus of the flowering plant family, Cannabaceae. There are three major species of the genus Cannabis: Cannabis sativa, Cannabis indica, and Cannabis ruderalis.
Within these three species there are substantial variations in tetrahydrocannabinol (THC) content, which is the bioactive agent that produces the “high” associated with cannabis ingestion. For example, the variations of cannabis that contain 0.3% or less THC by dry weight are called hemp, whereas the term marijuana refers to variations of cannabis that contain more than 0.3% THC by dry weight.
CBD can be produced from the leaves and flowers of either hemp or marijuana. However, there are important compositional and legal differences here. From a composition standpoint, hemp-derived CBD contains high levels of CBD (cannabidiol) and BCP (beta-caryophyllene), neither of which are psychoactive or cause an altered sensory experience, e.g. the “high” associated with marijuana, and have <0.3% THC. Conversely, marijuana-derived CBD contains mainly THC and lower levels of CBD. White 2019
Is CBD Legal?
With respect to the legal differences, things get interesting in a hurry! The 1970 Controlled Substances Act originally made it illegal to grow and sell any type of cannabis (including both hemp and marijuana) in the United States. All cannabis products were deemed “Schedule I” drugs, which are defined as having a high potential for abuse and have no currently accepted medical use.
It wasn’t until the 2014 Agricultural Act where hemp and marijuana were legally distinguished based on the THC content limits described above, which made it legal for hemp to be grown and distributed in the United States for “research purposes” only. An additional important legal ruling happened in 2014: the Rohrabacher-Farr amendment, which allowed individual states to implement their own rules about cannabis use and distribution, provided it wasn’t transported across state lines. Thus, it was still illegal to introduce any supplement or food containing cannabis, including hemp-derived CBD, into interstate commerce. Agricultural Act of 2014 Mead 2017
Subsequently, many Cannabis products were available for sale in select states up until 2018, when the Agricultural Act of 2018 or “Farm Bill” was passed. This bill now made hemp and hemp-derived products (including CBD Oil) legal for sale across states. VanDolah 2019 As a reminder, hemp is defined as a Cannabis variety that contains <0.3% THC by dry weight whereas marijuana is a variety of Cannabis that contains >0.3% THC. Thus, even if there was a marijuana-derived CBD oil product that contains <0.3% THC after extensive refinement processes, it would still be illegal based on the current laws. In summary, hemp-derived CBD is legal across the nation, but marijuana-derived CBD continues to be illegal regardless of THC content.
Finally, from a medico-legal standpoint there is a huge mess in the supplement industry right now. Under the current Federal Food, Drug, and Cosmetic act, any product (other than a food) that is intended to affect the structure or function of the body of humans or animals, is considered a drug. FDA 2019 At present, there are less than a handful of FDA-approved products derived from Cannabis:
- Cannabidiol (Epidiolex) is pure 100% CBD that is used to reduce seizure frequency associated with two rare, congenital seizure disorders: Lennox-Gastaut and Dravet syndromes. Epidiolex costs about $13-15 per milliliter and is distributed in 100mL containers, which cost about $1300-1500.
- Dronabinol (Marinol and Syndros) is a drug that contains synthetic THC and is FDA-approved for treating weight loss in AIDS, as well as chemotherapy-associated nausea and vomiting.
- Nabilone (Cesamet) is another synthetic THC drug that is used for nausea and vomiting associated with chemotherapy.
Despite these relatively narrow therapeutic indications, there are a great number of CBD-containing products being marketed for uses including sleep aids, pain relief, stress reduction, improved recovery from exercise, or as Beam CBD’s advertisements claim, “Better everything.” These claims are at odds with the current Federal Food, Drug, and Cosmetics Act and as such, the FDA has sent warning letters to over 20 different supplement companies in 2019 alone due various infractions such as improper claims, concentrations of ingredients, or contaminants found upon testing. FDA 2019
For example, in 2016 a multi-center study purchased 84 commercially available CBD products from 31 different companies via the Internet. These products were tested three times each to determine the average concentration of CBD in each product, the accuracy of the ingredients listed on the label, and whether or not there was any THC in the product. The results were concerning:
- The listed CBD concentrations were accurate in only 31% of products. 43% were overdosed relative to what the label stated and 26% were underdosed.
- The labels were found to be accurate in only 12.5% of vaporized products, 25% of tinctures, and 45% of oils.
- THC was found in 21% of samples, with an average concentration of 0.45 mg/mL. Of note, inhaling 2-3mg or ingesting 5-20mg of THC can cause the “high” associated with marijuana use. Bonn-Miller 2017
In summary, while the FDA-approved drugs that are derived from Cannabis have specific medical indications and FDA-enforced quality control and safety guidelines that must be maintained, the commercially available CBD products raise serious concerns about their safety and efficacy. When coupled with their wide-spread usage – 14% of Americans report using a CBD product – this may lead to unexpected problems.
How Does CBD Work?
The cannabis plant contains over 500 different chemicals, which are called cannabinoids. The main cannabinoids derived from the hemp varieties of Cannabis are cannabidiol (CBD) and beta-caryophyllene (BCP). Unfortunately, there isn’t much clinical research on what CBD or BCP does in the human body, as most of the existing research has focused on THC. Here’s what we know at present:
- The Endocannabinoid System (ECS) is present throughout the body including the brain, nerves, skin, bone, muscle, GI tract, and most of our major organs. It is involved in a wide variety of different processes including appetite, pain, mood, memory, sleep, etc. Witkamp 2014 Acharya 2017
- There are two main cannabinoid receptors, CB1 and CB2. CB1 receptors are widely distributed in most tissues, with the highest concentration in the brain. In contrast, CB2 receptors are concentrated in white blood cells and many organ systems such as the heart, liver, GI tract, and more. CB2 receptors are also located in the brain, but at much lower concentrations than CB1 receptors. Zou 2018
- The body naturally produces its own endocannabinoids, e.g. anandamide and 2-arachidonylglycerol, to modulate the ECS.
- Cannabinoids from plants, e.g. THC, CBD, and BCP, also act on the CB1 and CB2 receptors. THC directly binds to CB1 and BCP binds directly to CB2. It appears that CBD doesn’t bind to either, yet stimulates both through a mechanism that isn’t yet well established. Zou 2018
Overall, CBD likely affects the ECS using CB1 and CB2 receptors as well as other pathways that haven’t yet been established, which may be even more important. For example, studies on the FDA-approved drug Epidiolex suggest that the anti-seizure effect of CBD is not mediated through its effects on cannabinoid receptors, though the exact mechanism of action remains unknown. VanDolah 2019
How is CBD Administered?
Commercial preparations of CBD can be administered in a number of different ways such as by mouth from a pill, through the oral mucosa via a mist, spray, or drops, via the lungs through an inhaled product, or transdermally through a cream or salve. While there was a single study in the 1980’s where CBD was administered in humans via an IV, there are no commercial or prescription formulations of CBD that can be given intravenously. Ohlsson 1986
CBD appears to be well-absorbed orally via a pill, drops placed on or under the tongue, or via a mist sprayed into the mouth. Pills or capsules tend to increase blood levels faster and to higher levels than similarly-dosed drops or aerosolized CBD mists. Blood levels of CBD trend with the amount given in a dose-dependent fashion, e.g. the higher the dose, the higher the concentration of CBD in the blood, though this tends to level off at higher doses. CBD contained within a cigarette or nebulizer has greater bioavailability compared to orally-administered CBD, at 31% compared to 6%. Zhornitsky 2012 Additionally, consuming a meal one hour after oral administration of CBD ingestion tends to increase the absorption and the amount of time the CBD is detectable in the blood. Millar 2018
However, topically-applied CBD preparations do not have any human data indicating they are absorbed effectively. Rather, one mouse and one rat study show absorption of CBD gel through the skin. Rat skin is indeed similar to human skin from an anatomical standpoint. For example, the outer layer of the skin – the stratum corneum – is 18 micrometers thick in both the rat and the human. Additionally, the whole skin is about 2.09 millimeters thick in the rat and about 2.58 millimeters thick in humans. Jung 2015 Nevertheless, human data showing how much CBD is absorbed from topical CBD application are currently lacking.
What Does the Existing Data on CBD Say?
Unlike social media where CBD is often touted as a panacea, the current data on CBD’s effects on humans are restricted to seizure disorders, psychosis, pain, Parkinson’s disease, and anxiety. With the focus of this month’s Research Review being on the effect of CBD on anxiety, we’ll review some the other conditions first and save the anxiety discussion for later.
Seizures
At present, the only FDA-approved indication for CBD is to treat drug-resistant seizures. Four, multicenter, double-blind, placebo controlled trials show that prescription CBD, Epidiolex, reduced the frequency of seizures by about half compared to placebo. Devinsky 2017 Devinsky 2018 Devinsky 2016 Hess 2016 It is important to note that the population studied was primarily made up of those with either Lennox-Gastaut or Dravet Syndrome, which are relatively rare conditions that present during childhood and typically arise from genetic disorders. Nevertheless, pharmacologic-grade CBD administration has some solid evidence for these conditions.
Pain
In contrast to the well-controlled data on seizures, the evidence looking at CBD’s effects on pain is scant and of very low quality.
For example, a study looked at twelve young women (age 12-24 years) who received the Human Papilloma Virus (HPV) vaccine and subsequently developed dysautonomic syndrome. This is thought to be an autoimmune condition that is characterized by pain, (e.g. headaches, joint and muscle pain, etc.), although its connection to the HPV vaccine is tenuous at best. For example, the largest study evaluating the safety of the HPV vaccine compared ~300,000 young women who received the vaccine to ~700,000 who did not. Those who received the vaccine showed no increases in autoimmune or neurological diseases in those who did not. Dahlstrom 2013
In any case, these 12 women with symptoms of dysautonomic syndrome were given CBD oil as drops under the tongue daily over the course of 3 months. During this time, two women dropped out due to adverse events and another two stopped taking the CBD due to lack of improvement. The remaining 8 individuals showed a statistically significant reduction in body pain and both physical and social functioning compared to their baseline scores at the beginning of the test. Palmieri 2017 That said, this is a very small study that additionally had no placebo-controlled group or a no-treatment group to compare these outcomes to. Did the CBD do anything specifically, or did the condition just run its natural course? This study design makes it impossible to say.
In fact, there are only two randomized, double-blind, placebo-controlled trials assessing CBD’s effect on pain in humans. Researchers out of the UK looked at 24 patients with one of the following conditions: multiple sclerosis (n=18), spinal cord injury (n=4), brachial plexus damage (n=1), and limb amputation due to neurofibromatosis (n=1). Each patient received CBD, THC, CBD + THC, or placebo for 2 weeks during each phase of the trial in a crossover fashion. In other words, each participant would take one agent for 2 weeks before crossing over to another agent for the next 2 weeks, and repeat the process until they’d spent 2 weeks taking each of the possible combinations. Thus, each subject served as their own placebo-matched control.
On each of the last 7 days of each phase, the subjects reported their pain, muscle spasms, bladder function, muscle spasticity, and coordination based on a visual scale that ranged from 0 to 100 (0 = worst to 100 = best). An average score was generated for each patient in each phase and when all the results were in, the CBD group had slightly better pain control: 54.8 in the CBD group compared to 44.5 in the placebo group (again recall 0 = worst and 100 = best in this study). There were no other statistically significant differences between CBD and placebo in any of the other outcomes measured. In addition to the wildly different populations studied here, only 12 of the patients completed the pain assessment for all of the possible interventions. Wade 2003
More recently, researchers out of the Netherlands also used a crossover study design where 20 subjects with fibromyalgia received one of four treatments: THC, THC +CBD, CBD, or placebo. Each week, the subjects got one of the treatments for a single dose and then rated their pain. There were no differences between CBD and placebo on any of the pain-related outcomes. van de Donk 2019
Overall, the present data do not clearly support the use of CBD products for pain management. In fact, there seem to be more review articles on CBD than actual trials! Going forward, we’d like to see adequately-powered, randomized, double-blinded, placebo-controlled trials on CBD for specific indications in humans. Right now, the data are severely lacking.
Parkinson’s Disease
A single trial investigated the efficacy of CBD on patients with Parkinson’s disease and found no benefit in the movement aspects associated with the disorder such as tremor, slow movement, rigidity, and postural instability. Additionally, there was no difference between those receiving CBD or placebo in the Unified Parkinson’s Disease Questionnaire-39, a validated questionnaire used to assess the severity of Parkinson’s disease in the clinical setting (mainly research). Interestingly, a small subset (n=4) of these subjects had Parksinson’s disease-associated sleep behavior disorder did see a reduction in frequency of this condition, which is characterized by nightmares and the muscles being rigid instead of relaxed during sleep. Chagas 2014 Unfortunately, there are no randomized, controlled trials investigating how CBD may affect other sleep conditions such as insomnia or sleep-phase disorders.
Psychosis
At present, there are 3 randomized-controlled trials looking at the efficacy of CBD in patients diagnosed with schizophrenia, a psychiatric condition involving chronic or recurrent psychosis. While all demonstrate an improvement in the symptoms of schizophrenia over time, the trials are small (<100 patients) and the differences between placebo and CBD or traditional antipsychotics and CBD were very small. White 2019
Anxiety
There are a lot of claims that CBD oil reduces anxiety and/or calms individuals down. Given the prevalence of these claims, I want to take a deeper dive into the evidence here, starting with a 2017 paper on anxiety from researchers in Brazil. The researchers selected the Test of Speaking in a Real Situation (TPSRS) to generate anxiety. In this study, subjects had 1-minute to prepare a 2-minute speech on “the conditions of one public service of your city” and then present their speech with the other subjects serving as the audience.
Based on previous animal studies, the researchers hypothesized that there would be a “inverted U-shaped” dose-response curve between CBD and effect. An inverted U-shaped dose-response curve looks like a bell curve and suggests that moderate doses, but not high or low doses, will produce the biggest effects. Thus, the goal of the study was designed to test the hypothesis that increasing doses of CBD would produce anxiolytic effects in an inverted U-shaped dose-response pattern in healthy volunteers submitted to the TPSRS.
The researchers recruited 60 men and women aged 18-35 years, with no history of past or current anxiety, psychiatric condition, substance use disorder, or major medical condition. Each subject had their propensity for anxiety measured using the Spielberger State Trait Anxiety Inventory (STAI-trait), which consists of 20 questions about how often someone feels a particular way, e.g. calmed down, safe, tense, annoyed, stunned, upset, etc. The individual can respond with never (1 point), sometimes (2 points), frequently (3 points), or almost always (4 points). Emotions associated with being anxious are scored positively, whereas emotions associated with being calm are scored negatively. Higher scores tend to predict a higher propensity towards anxiety.
The subjects were randomly separated into 5 groups of 12 subjects, each matched for gender (6 men and 6 women), age (average age 22 years), BMI (average 22-23), and STAI-trait score (41-46). The 5 groups received the following:
- CBD oil 100 mg (99.6% pure CBD powder + corn oil)
- CBD oil 300 mg (99.6% pure CBD powder + corn oil)
- CBD oil 900 mg (99.6% pure CBD powder + corn oil)
- Clonazepam 1 mg
- Placebo (corn oil)
For reference, clonazepam is a benzodiazepine that is FDA-approved for panic disorder and seizure disorders. There is ample data supporting the use of benzodiazepines, including clonazepam, to reduce each of the three components of panic disorder (attack frequency, anticipatory anxiety, and avoidance). With that said, there is a substantial risk of abuse, addiction, and side effects with benzodiazepines. Of note, the 1 mg dose here is the maximum dose recommended for treating panic disorder per the FDA.
Psychological measurements of anxiety were obtained using the Visual Analog Mood Scale (VAMS), where the individuals rated how they felt by selecting a point on a 100mm line between two pictographic representations of emotions (see Figure 1). Anxiety was assessed by the items calm–excited, relaxed–tense, and tranquil–troubled, whereas sedation was assessed using the items alert–drowsy and attentive–dreamy.
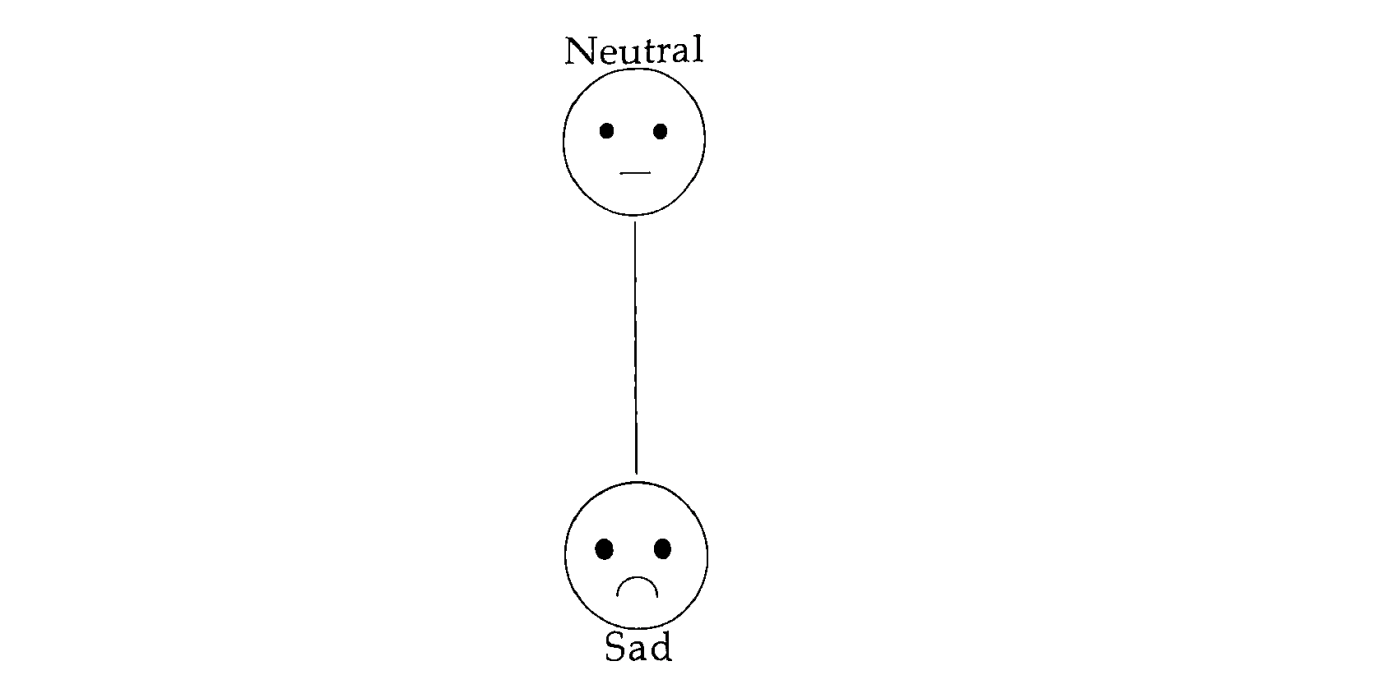
Figure 1: Pictographic representation of neutral-sad emotions. The line between the two pictures is 100mm and test subjects would mark where they currently felt along the line between the two pictures. The researchers then measured the distance between to determine how anxious and sedate the subjects where during the test.
The VAMS was administered four times in total, once at baseline when the medication or placebo was taken (0 minutes), once prior to the instructions for the public speaking test (80 minutes), once during the speech (153 minutes), and again one hour after the speech (216 minutes). Blood pressure and heart rate were also assessed at these times. Unfortunately, the authors did not provide the raw data for the VAMS scores or physiological data at any point during the experiment.
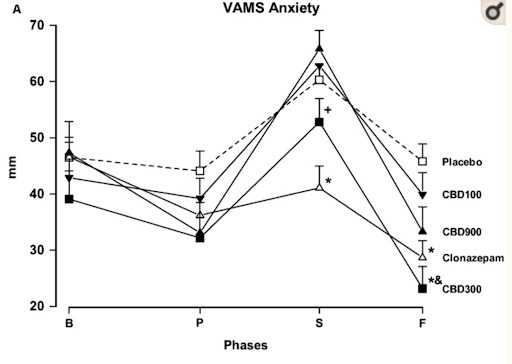
Figure 2: VAMS-Anxiety results from the experiment. “B” stands for baseline, which is when the medication or placebo was administered. “P” stands for pre-stress, which is prior to when the instructions for the speech test were given. “S” stands for speech, which were measurements taken in the middle of the public speech. “F” stands for final, which were measurements taken one hour after completing the speech test. Asterisks denote statistically significant findings compared to the placebo group. “&” indicates a significant difference compared to the CBD-100 group.
Based on the above graph and the author’s report, we can see that those receiving 300 mg of CBD or 1 mg of clonazepam both had significantly less anxiety during the speech and one hour after the speech as measured by the VAMS. It also appears that clonazepam was significantly better at reducing anxiety than 300 mg of CBD oil during the speech, but not one hour later. With respect to sedation, only clonazepam showed a significant increase in sedation compared to the other groups, however there were no differences between placebo and any of the groups who received CBD (see Figure 3). Finally, there were no significant differences in anxiety between placebo and 100 or 900 mg of CBD at any time point.
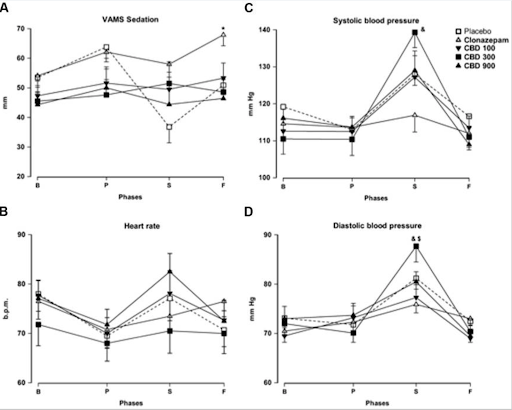
Figure 3: VAMS-Sedation and physiologic results from the experiment. “B” stands for baseline, which is when the medication or placebo was administered. “P” stands for pre-stress, which is prior to when the instructions for the speech test were given. “S” stands for speech, which were measurements taken in the middle of the public speech. “F” stands for final, which were measurements taken one hour after completing the speech test. Asterisks denote statistically significant findings compared to the placebo group. “&” indicates a significant difference compared to the CBD-100 group. “$” indicates a significant difference to the CBD-100 group.
The physiological data is shown in Figure 3. Of note, 300 mg of CBD had significantly higher systolic blood pressure than clonazepam during the speech phase only, but was not significantly different compared to 100 mg of CBD, 900 mg of CBD, or placebo during this time. There were no significant differences in systolic blood pressure at any other time. Additionally, there were no significant differences in heart rate between any of the medications or placebo at any time. Finally, the diastolic blood pressure was significantly higher during the speech phase in those receiving 300 mg of CBD compared to 100 mg of CBD or clonazepam, but not compared to those receiving 900 mg CBD, or placebo. There were no significant differences in diastolic blood pressure at any other time.
What Is the Take-home Message?
Overall, this data suggests that there may be an inverted U-shaped dose-response curve for CBD to reduce anxiety during public speaking. While 300 mg of CBD and clonazepam both reduced as measured by the VAMS test, lower (100 mg of CBD) or higher (900 mg of CBD) did not reduce anxiety. Of course, this assumes these small changes in the VAMS test are clinically significant. At present, there isn’t an established minimal clinically important difference (MCID) for this test. Thus, it’s hard to feel confident about these findings.
With that being said, this may be misleading because all of the subjects were of similar size (BMI 22-23) and instead, the appropriate dose for CBD to reduce anxiety during public speaking may be weight-based like it is for the seizure disorders discussed earlier (2.5 mg/kg twice per day). White 2019
Of note, the study reviewed this month is likely underpowered to detect significant differences between CBD dosing, given that there were only 12 subjects in each group. Additionally, there were no statistically significant differences between clonazepam and CBD 300 mg with regards to reducing anxiety, but only the clonazepam increased sedation. Finally, those receiving 300 mg of CBD both had a significant increase in systolic and diastolic blood pressure during the speech phase, which was not seen in the other treatment arms.
Other studies investigating how CBD affects anxiety outside of public speaking use single-dose CBD, have very small sample sizes, use a wide variety of different CBD doses and routes of administration, and use individuals without anxiety. This makes it difficult to determine what, if any, the chronic impact of CBD on anxiety is, the optimal dose and route of administration, or if CBD can be effective in those diagnosed with a particular anxiety condition. For example, we’d expect those with a diagnosed anxiety condition to potentially benefit more from an anxiolytic (anxiety-reducing) drug than individuals without anxiety. Martin-Santos 2012 Arndt 2017 Hundal 2018
Overall, the data on CBD outside of reducing seizure frequency in those with drug-resistant seizure disorders does not support the notion that CBD is particularly useful for pain, anxiety, neurological conditions such as Parkinson’s disease, or psychiatric conditions like schizophrenia. There are no data at this time regarding CBD and the many claims made about it on the Internet such as its effect on exercise performance or recovery and sleep quality or duration in otherwise healthy individuals. There is also a significant concern for adverse reactions including diarrhea (9-20%), anemia (30%), liver injury (8-17%), and interactions with other drugs. Brown 2019 Taken together with the concerns over CBD quality given that less than 1/3 are accurately labeled, I don’t think that CBD should be viewed as a panacea, but rather should be approached with caution until better data emerge.
References:
- Brenan, Megan. “14% Of Americans Say They Use CBD Products.” Gallup.com, Gallup, 7 Oct. 2019, news.gallup.com/poll/263147/americans-say-cbd-products.aspx.
- Corroon J, Phillips JA. A Cross-Sectional Study of Cannabidiol Users. Cannabis Cannabinoid Res. 2018;3(1):152–161. Published 2018 Jul 1. doi:10.1089/can.2018.0006
- Franck, Tom. “Top Cannabis Analyst on Wall Street Raises Sales Forecast, Names Top 2019 Picks.” CNBC, CNBC, 8 Jan. 2019, www.cnbc.com/2019/01/08/top-weed-analyst-on-wall-street-raises-sales-forecast-names-top-picks.html.
- Jung E.C., Maibach H.I. Animal models for percutaneous absorption. J. Appl. Toxicol. 2015;35:1–10. doi: 10.1002/jat.3004
- Future, Market Research. “CBD Oil Market Analysis 2019: Hemp Oil and Medical Marijuana to Capture the Largest Share in CBD Oil Industry |.” Medgadget, 27 Nov. 2019, www.medgadget.com/2019/11/cbd-oil-market-analysis-2019-hemp-oil-and-medical-marijuana-to-capture-the-largest-share-in-cbd-oil-industry.html.
- Agricultural Act of 2014, HR 2642, 113th Cong, 2nd Sess (2014). US Government Publishing Office website, https://www.gpo.gov/fdsys/pkg/BILLS-113hr2642enr/pdf/BILLS-113hr 2642enr.pdf
- White, C. M. (2019). A Review of Human Studies Assessing Cannabidiol’s (CBD) Therapeutic Actions and Potential. The Journal of Clinical Pharmacology. doi:10.1002/jcph.1387
- VanDolah, H. J., Bauer, B. A., & Mauck, K. F. (2019). Clinicians’ Guide to Cannabidiol and Hemp Oils. Mayo Clinic Proceedings. doi:10.1016/j.mayocp.2019.01.003
- Bonn-Miller MO, Loflin MJ, Thomas BF, et al. Labeling accuracy of cannabidiol extracts sold online. JAMA. 2017;318: 1708-1709.
- Commissioner, Office of the. “FDA Regulation of Cannabis and Cannabis-Derived Products: Q&A.” U.S. Food and Drug Administration, FDA, www.fda.gov/news-events/public-health-focus/fda-regulation-cannabis-and-cannabis-derived-products-including-cannabidiol-cbd.
- Witkamp R, Meijerink J. The endocannabinoid system: an emerging key player in inflammation. Curr Opin Clin Nutr Metab Care. 2014;17(2):130-138.
- Acharya N, Penukonda S, Shcheglova T, Hagymasi AT, Basu S, Srivastava PK. Endocannabinoid system acts as a regulator of immune homeostasis in the gut. Proc Natl Acad Sci U S A. 2017;114(19):5005-5010.
- Zou S, Kumar U. Cannabinoid Receptors and the Endocannabinoid System: Signaling and Function in the Central Nervous System. Int J Mol Sci. 2018;19(3):833. Published 2018 Mar 13. doi:10.3390/ijms19030833
- Millar, Sophie A., et al. “A Systematic Review on the Pharmacokinetics of Cannabidiol in Humans.” Frontiers in Pharmacology, vol. 9, 26 Nov. 2018, doi:10.3389/fphar.2018.01365.
- Devinsky O, Marsh E, Friedma D, Thiele E, et al. Cannabidiol in patients with treatment-resistant epilepsy: an open-label interventional trial. Lancet Neurol. 2016;15:270-278
- Hess EJ, Moody KA, Geffrey AL, et al. Cannabidiol as a new treatment for drug-resistant epilepsy in tuberous sclerosis complex. Epilepsia. 2016;57:1617-1624.
- Devinsky O, Cross JH, Laux L, et al. Trial of cannabidiol for drug-resistant seizures in the Dravet syndrome. N Engl J Med. 2017;376:2011-2020.
- Devinsky O, Patel AD, Cross JH, et al. Effect of cannabidiol on drop seizures in the Lennox-Gastaut syndrome. N Engl J Med. 2018;378:1888-1897.
- Arnheim-Dahlström L, Pasternak B, Svanström H, Sparén P, Hviid A. Autoimmune, neurological, and venous thromboembolic adverse events after immunisation of adolescent girls with quadrivalent human papillomavirus vaccine in Denmark and Sweden: cohort study. BMJ. 2013;347:f5906. Published 2013 Oct 9. doi:10.1136/bmj.f5906
- Palmieri B, Laurino C, Vadala M. Short-term efficacy of CBD- ` enriched hemp oil in girls with dysautonomic syndrome after human papillomavirus vaccination. IMAJ. 2017;19:79-84.
- Wade DT, Robson P, House H, et al. A preliminary controlled study to determine whether whole-plant cannabis extracts can improve intractable neurogenic symptoms. Clin Rehabil. 2003;17:21-29.
- Chagas MH, Eckeli AL, Zuardi AW, et al. Cannabidiol can improve complex sleep-related behaviours associated with rapid eye movement sleep behaviour disorder in Parkinson’s disease patients: a case series. J Clin Pharm Ther. 2014;39(5):564- 566.
- van de Donk T, Niesters M, Kowal MA, Olofsen E, Dahan A, van Velzen M. An experimental randomized study on the analgesic effects of pharmaceutical-grade cannabis in chronic pain patients with fibromyalgia. Pain. 2019;160(4):860–869. doi:10.1097/j.pain.0000000000001464
- Zhornitsky S., Potvin S. (2012). Cannabidiol in humans-the quest for therapeutic targets. Pharmaceuticals (Basel). 5 529–552. 10.3390/ph5050529
- Martin-Santos R, Crippa JA, Batalla A, et al. Acute effects of a single, oral dose of d-9-tetrahydrocannabidiol (THC) and cannabidiol (CBD) administration in healthy volunteers. Curr Pharm Design. 2012;18:4966-4979.
- Arndt, DL, Harriet de Wit H. Cannabidiol does not dampen responses to emotional stimuli in healthy adults. Cannabis and Cannabinoid Res. 2017;2(1):105-113.
- Hundal H, Lister R, Evans N, et al. The effects of cannabidiol on persecutory ideation and anxiety in a high trait paranoid group. J Psychopharmacol. 2018;32:276-282
- Brown JD, Winterstein AG. Potential Adverse Drug Events and Drug-Drug Interactions with Medical and Consumer Cannabidiol (CBD) Use. J Clin Med. 2019;8(7):989. Published 2019 Jul 8. doi:10.3390/jcm8070989