This is part two of the multi-part Hunger Games series. Check out part one here.

Ghrelin, as mentioned before is made by cells in the stomach and released in the bloodstream in ever-increasing amounts before meals. The longer one goes between meals, the more ghrelin will be released and the more stimulation there will be for hunger. Directly after a meal ghrelin drops off. Ghrelin acts on the hypothalamus on both the agouti-related protein and neuropeptide Y releasing cells, showing the redundancy and important nature of its activity there. It also acts directly on the stomach and modifies the stomach’s sensitivity to distension. In allowing the stomach to become more distended by desensitizing the vagus nerve, which is responsible for sensing the fullness of the stomach, it allows for higher amounts of food to be taken in.
Since ghrelin induces a person to eat it might be easy to point the finger at this hormone and claim it is responsible for over-eating, and thus, obesity. However, a study published in The New England Journal of Medicine (NEJM) showed the ghrelin levels are actually lower in obese individuals compared to their lean counterparts. Furthermore, those with anorexia nervosa have high amounts of circulating ghrelin, thereby suggesting it’s not the only issue for those with weight management issues. Ghrelin also is involved in regulating fat oxidation (burning) and fat accumulation in the body. When ghrelin levels are high centrally, in the hypothalamus- like when you’re hungry, fat burning slows down and fat accumulation machinery gears up for the influx of calories the body thinks is coming. Perhaps this is part of the mechanism behind why “starving” oneself is not a good diet strategy. One last interesting thing about ghrelin is that it requires a specific enzyme, GOAT, which helps activate ghrelin basically. It appears that medium chain triglycerides (MCTs), such as those found in butter and coconut oil/milk, are acted upon by this enzyme directly- leading to immediate ghrelin signaling. This information tells the hypothalamus that calories are coming and ghrelin can cool it with the hunger pangs. Chalk one up for MCT’s!

Neuropeptide Y (NPY) is another appetite stimulant released from the hypothalamus and is also involved in energy usage, generally decreasing it. Put simply, it increases caloric intake and decreases caloric expenditure. NPY also has a tendency to increase the storage of food as fat. A chronic increase in NPY has been implicated as one of the underlying causes of obesity, as the stress hormone cortisol tends to increase NPY while also decreasing the regulation of NPY synthesis and release. What this means is that high levels of stress from things like lack of sleep, constant starvation/undereating, stressful job or personal situations, etc. might all be keeping NPY levels elevated, which is a known appetite stimulant and fat accumulation promoter. Leptin inhibits NPY release, however, so perhaps leptin dysregulation is involved in NPY running amuck. Also, anorexic patients have high levels of NPY, implicating that they are driven to eat and are hungry, however they managed to overcome this via their psychology. So perhaps people that comment that they are hungry while dieting might also be able to overcome the hunger pangs.

Many studies associate NPY with carbohydrate-rich food preference, that NPY might increase carbohydrate intake above baseline. An American Journal of Physiology study done with rats showed that when rats had higher NPY levels, either through food deprivation or when injected with extra NPY, that the rats consumed more sugary (sucrose and corn sugar) carbohydrates. Conversely, when the rats were allowed to eat freely they preferred a higher-fat and lower-carbohydrate style of chow. Whether or not this lends any credence to the idea of eating more frequent meals to prevent NPY and ghrelin levels from creeping up to high, as less time between meals might theoretically result in a lower stimulus to fill up on high-energy foods, has yet to be determined definitively in the literature.
Interestingly, there appears to be connections between sleep levels and food intake. Sleep deprivation is linked to increased ghrelin levels, increasing the drive to acquire food, and increased NPY levels. High NPY levels suppress sleep and promote feeding according to the journal, Aging, which might affect insulin sensitivity and function as sleep deprivation is clinically associated with metabolic syndrome and diabetes mellitus.
The last appetite stimulant we’ll discuss here is anandamide, which is a naturally occurring substance in the brain that binds to the cannabinoid receptors in the brain. Yes, these are the same receptors that marijuana affects and you can probably guess what anandamide does. An example that might help clarify anandamide’s activity is the mouse model. Mice who are given extra anandamide eat more than mice that don’t and mice whose cannabinoid receptors have been knocked out, eat less. Perhaps it’s no surprise then, that some seriously ill patients like those with AIDS find marijuana to be a potent appetite stimulus. A drug, Rimonabant, was marketed for a short time in Europe as an appetite suppressant as it blocked anandamide and a similar compound from binding to the cannabinoid receptors in the brain. Due to side effects, however, it was pulled from the market and never made it to the United States.
In summary, ghrelin and NPY tend to be the most potent appetite stimulators physiologically. While they do not have a totalitarian role in feeding because there are certainly psychological factors involved, their functions need to be understood and respected. Certain dietary and lifestyle changes may change these hormones’ concentrations in the body, and these too must be appreciated. A carbohydrate-restricted diet coupled with improved sleep quality/quantity, for instance, might lower circulating ghrelin and NPY to a degree and decrease hunger, as the body becomes increasingly reliant on oxidizing (burning) fatty acid for fuel, both from dietary sources and the body’s own fat stores. Consider this, a 180lb male with 15% body fat has 27 pounds, or 94,500 calories of fat available to be burnt before literally running on empty. Now the body certainly has the ability to use both sugar and fat as fuel when creating energy, however it will prefer sugar (glucose) as long as it’s available in substantial amounts. If a person were to embark on a lower carbohydrate diet, he or she would go through a transitional period where their energy producing cell parts, the mitochondria, would convert their machinery to be more efficient at burning fat. This can take anywhere from days to a couple weeks to occur, however it will happen because we all have the capability to burn fat, as it’s an evolutionary adaptation to utilizing various energy sources to give us fuel. When the body’s cellular machinery becomes more adept at burning fat, and there’s tens of thousands of calories lying in wait to be oxidized, the body does not sense that it’s running low on fuel. This is especially true when compared to a person who is used to running on sugar primarily. Ever notice that energy crash after a carbohydrate-rich meal? That’s because the influx of broken down carbohydrates, sugar or glucose more appropriately, enters into the blood stream and then has the be shuttled away to keep blood sugar levels in check. When blood sugar decreases, as there is often an overshoot to removing sugar from the blood, hunger pangs strike again. Conversely, if a body is more akin to running on fatty acids for fuel, this never occurs, and thus the drive to feed can be mitigated. Now certainly ghrelin and NPY levels will still increase if a person is under fed or is starving themselves, but numerous studies have indicated that a punctuated lower carbohydrate approach can be dramatically effective at not only weight loss, but reducing hunger and increasing compliance with the nutritional protocol. We’ll discuss these towards the end of this article.

Leptin is an appetite suppressant that is manufactured by the cells of the fat tissue, the adipocytes, and is a long-term inhibitor of food intake. It also increases fat release from fat tissue and metabolic rate. The circulating level of leptin in the body is directly proportional to the amount of fat in the body, which means that fatter persons have more circulating leptin compared to leaner people. In mice, a mutation in the gene for leptin or its receptor causes them to become grossly obese, as we’d expect because they have no inhibition to cease food intake. When these mice where given injections of leptin they lost their excess fat and returned to normal body weight. Leptin counteracts NPY, anandamide, and other appetite stimulants at the level of the hypothalamus.
Leptin has a few other cool little functions, like being involved in sex steroid (estrogen and testosterone) regulation. Leptin helps signal gonadotropin-releasing hormone (GnRH) from the hypothalamus, which is involved in the menstrual cycle. Women who have very low body fat, and thus not enough circulating leptin might cease to menstruate for this reason.
The New England Journal of Medicine published a case report on a 9-year-old girl who was born with a gene mutation in the gene for leptin, i.e. she produces no leptin on here own. She started out weighing 208lbs (123lbs of it fat) and was given leptin injections for a year. At the end she had lost 36lbs total, most of it being fat, and her appetite and food intake had decreased. While this is certainly an improvement, she has another 70lbs of fat to lose to be lean and one might think that the leptin injections would’ve taken her much farther in a year. On the other hand, the study said nothing of her growth (height, bone density, etc.), which might be relevant in a 9-year-old girl. Still, it seems as though leptin is a very important part of this whole system, but perhaps not the one and only important factor. The Journal of Clinical Endocrinology published a review of clinical trials involving people with this same mutation being treated with leptin and found that only the highest doses produced statistically significant weight loss.

The more fat someone carries the more circulating leptin, which should increase his or her metabolic rate, fat oxidation, and decrease hunger but apparently does not. Much research has been done on this very topic, and in short, it appears that the leptin either does not reach the hypothalamus or it does but the hypothalamus doesn’t recognize it. This is called leptin resistance. Rats who have been genetically modified to not have the leptin receptor, Zucker fatty rats, present with obesity, low metabolism, elevated hunger, and insulin resistance. This is classic leptin resistance as the rats produce plenty of leptin but cannot respond to it. This is similar to metabolic syndrome in Americans, which affects 24% of the population according the CDC. In a 2005 publication of the journal, Obesity Research, leptin levels (elevated) predicted impending development of metabolic syndrome. In other words, leptin resistance- from diminished response to leptin precedes development of obesity and insulin resistance (symptoms of metabolic syndrome). So what causes leptin resistance?
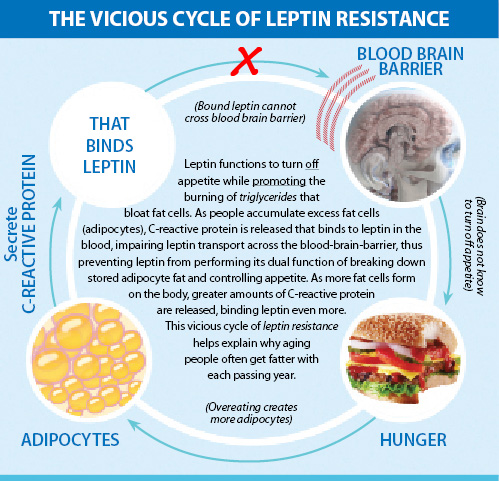
It appears that fructose and glucose intake along with inflammation of the hypothalamus are heavily involved in leptin resistance. Fructose is metabolized by the liver and will be converted to glycogen eventually to be stored in the liver. The liver stores glycogen that is to be slowly released during the times between meals to keep blood sugar levels normal. So when fructose is consumed in a meal the liver’s glycogen stores need to be topped off before anything else happens with the remaining fructose. After the glycogen stores are filled in the liver the excess fructose is metabolized into a triglyceride, palmitic acid mainly. Palmitic acid in high concentrations along with high triglycerides in general, have been associated with leptin resistance. The proposed mechanism is that this triglyceride as well as others interfere with the transmission of leptin across the blood-brain barrier, and thus leptin cannot act upon the hypothalamus correctly. Additional research has linked inflammatory markers like C-Reactive Protein (CRP) with leptin resistance, lending some credibility to this hypothesis. Causes of chronic inflammation can include stress, sleep deprivation, chronic infection or disease, as well as certain nutritional and lifestyle factors that we’ll discuss later.

Fructose intake has risen tremendously in the past 100 or so years mainly in the form of sweeteners and high-fructose corn syrup. Consider that artificial sweeteners were not used in the late 1800’s and that by 2005 we were consuming 142 pounds of the stuff per year per person! A study published in the American Journal of Clinical Nutrition found that just 4 weeks of fructose feeding in humans at a level of 1.5g/kg body weight increased leptin levels by 48%, while weight increases were not observed. So leptin levels went up while body fat levels stayed the same, thereby indicating the potential onset of leptin resistance. Lest you think that the dose of fructose in this study was crazy high, let’s do a little thinking about this. A 150-pound person (68kg) would be given about 100g of fructose a day. A large apple has about 15g of fructose in it whereas one super-sized coke from the clown has 56g of fructose. Could someone theoretically eat 6-7 apples a day? Sure, especially since everyone knows that fruit is super healthy for them, right? It’s probably more likely that someone would overdose on sodas and other artificially sweetened foods though. We’ll be talking more about leptin later for sure.

Insulin is a hormone released from cells in the pancreas in response to a rising level of blood sugar. It has many actions like stimulating skeletal muscles to absorb glucose and convert it to glycogen and take up amino acids (the broken down protein fractions) and convert them into muscle proteins. In the liver insulin also increases glucose uptake from the blood and glycogen formation while decreasing glycogen breakdown and gluconeogenesis (conversion of fats and protein into glucose). Insulin acts on fat tissue to increase the uptake of glucose (get the picture?) and increase the formation of fat. Finally, insulin acts on cells in the hypothalamus to reduce appetite.

Insulin levels are increased proportionally to blood sugar levels relative to each individual’s insulin sensitivity. Insulin levels are also increased by eating as we mentioned earlier with the cephalic insulin response being present before any digestions or absorption of food had occurred. Insulin levels can be greatly influenced by diet, lifestyle, and muscle mass as well. Chronically high levels of insulin (hyperinsulinemia) are one of the classic symptoms of type II diabetes and metabolic syndrome. Type II diabetes used to be referred to as adult-onset diabetes, as typically only adults got the disease. However 20% of all newly diagnosed cases are children so that nickname had to be dropped.
As mentioned earlier, blood glucose increases when digested carbohydrates are absorbed through the small intestine to the blood stream. When blood sugar increases insulin is released from the pancreas to stimulate muscle, liver, and fat cells to take up the excess sugar from the blood. Let’s focus on the effect of insulin on fat tissue, specifically the accumulation of fat. You see, fat tissue is in flux all the time and is constantly being released into and sequestered out of the blood. The net flux of fat at the end of the day will determine which way your body composition is going. If you sequester more fat than you burn, you’re going to increase your body’s fat stores and get fatter. The opposite is also true, although it should be noted that this effect is better visualized over the long term. A single day where the net flux of fat is INTO THE FAT TISSUE versus OUT OF THE FAT TISSUE is not a death sentence, but rather the totality of a week or series of days would provide a better picture of what the fat is doing.
Insulin affects this net flux by fixing free fatty acids (FFA) in the fat tissue into triglycerides. Once three FFAs are fixed within the fat tissue they are trapped until given a signal to come out, like when insulin’s opposing hormone glucagon comes out. When blood sugar decreases glucagon springs into action by signaling the fat tissue to release it’s triglycerides (after they get broken back down into FFAs) so that some of these fats can be used as fuel and others can get converted into new sugars in the liver. The liver makes new sugar to keep blood sugar on an even keel, so even when no dietary carbohydrates (or small amounts) are present in the diet the body can and will make sugar out of other materials it has lying around. Glucagon is one of the main drivers of this process, gluconeogenesis, as well as increased fatty acid oxidation (burning). Glucagon also seems to be increased by protein intake. Dr. Atkins and many before him realized that insulin and glucagon were very important hormones in fat regulation. Even before insulin’s discovery in 1922 by Banting and Macleod ketogenic and carbohydrate restricted diets were used in weight loss protocols in order to manipulate a particular fattening substance as they called it back in “the day”.

Low carbohydrate diets have become very popular in recent years and there’s been a lot of talk about controlling insulin levels so you don’t store any fat. The idea that insulin levels drop when blood sugar doesn’t increase AS much is certainly true. Normal blood sugar is between 80-120mg/dl and normal insulin levels are between 40-50 micro units/ml. During fasting blood sugar drops to about 65-75mg/dl and insulin to the 7-10 range and remember, this is with no ingestion of calories at all! A ketogenic diet, or one that is very low in carbohydrates, results in a blood sugar that is between 80-85 mg/dl and an insulin level of 20-30. You see, some fats and all proteins will cause insulin to rise to varying degrees. However, even with these transient insulin spikes the relative amount of insulin is much lower than if carbohydrates were a large staple of the diet. This is one of the reasons that low carbohydrate diets are so effective.

We said earlier that insulin was an appetite suppressant so how would lowering it (and making us hungrier) help us with fat loss? The insulin response to food is mainly geared around lowering blood sugar. Insulin’s activity on the hypothalamus, on the other hand, is transient in that it tells certain parts of the hypothalamus “Hey, we did it- we ate and are now dealing with the aftermath.” When insulin levels decrease it’s not like this is a stimulus to eat in and of itself. Most authorities on the subject state that blood sugar dropping is one of the main factors behind hunger. Remember the liver stores lots of glycogen that it slowly releases when blood sugar starts to drop, which is one of cortisol’s effects.
Peptide YY (PYY) is the last hormone we’ll talk about in relation to physiological regulation of hunger and eating habits. It is a hormone released from the ileum and colon in response to eating. Specifically, it tries to tell us to stop eating and also increases our CNS’s sensitivity to leptin. Protein causes the greatest release in PYY, followed by fats, and carbohydrates release only small amounts of PYY. Perhaps the typical ketogenic or carbohydrate-restricted diet, one moderate in protein and high in fat, causes such a large release of PYY to attenuate the activity of NPY and ghrelin and thus cause satiety.
In summary, it appears the ghrelin and neuropeptide Y are the primary stimulators of appetite and leptin, PYY, and insulin are the primary suppressors. It also appears the insulin is heavily involved in energy storage in fat tissue. Finally, it’s reasonable to assume that leptin and insulin resistance might both be at the heart of the weight gain and obesity. I’ll wait until after we talk about the psychological factors influencing dietary habits before tackling the practical aspect of all this.