Part III will focus on the specific effects of resistance training on youth athletes. As mentioned in the previous parts of this series, resistance training is effective for increasing performance and reducing the risk of injury in athletes. Here we will explore the mechanisms by which it may elicit these effects.
(Editor’s note: Click here for Part I or Part II. Click on the media player below if you want to listen to the narrated version of this text.)
There is a paucity of research directly related to tissue changes in adolescents due to the invasive nature of methods such as muscle biopsy, or exposure to ionizing radiation via measurement of bone mineral density. Many studies therefore use performance and functional outcomes as proxies for physiological adaptations, so while we cannot directly infer tissue-level adaptation from these results, it does add useful information with respect to program design. We will review the data that do exist for youth athletes, and point out when our understanding is extrapolated from data in the adult population.
For the global effects of resistance training Teixeira et al reviewed the effects of skeletal muscle loading (SML) on skeletal muscle repair (SMR). While there were not specific dosage parameters given for what constitutes “loading”, we can begin to distinguish the effects of loading versus inactivity in general terms.
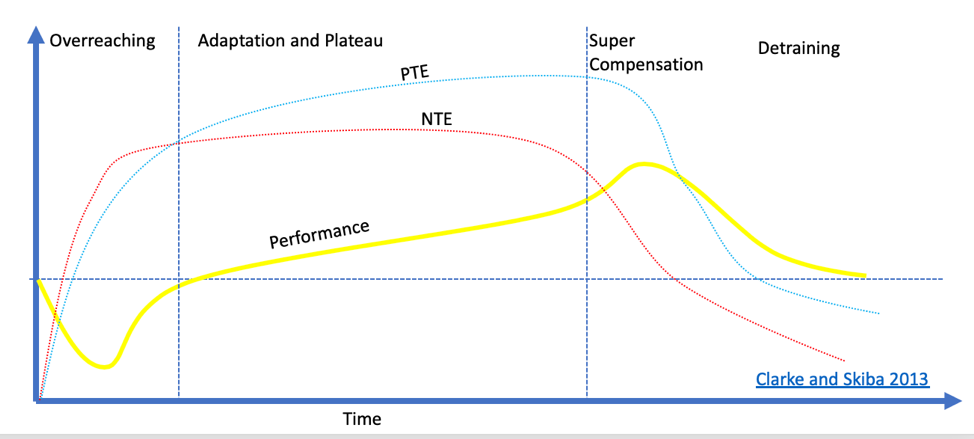
The above figure from Clarke and Skiba demonstrates the response to resistance training. Different forms of stimuli elicit different tissue adaptations. While increasing VO2max may be a Positive Training Effect (PTE) for swimming, decreased bone mineral density could be seen as a Negative Training Effect (NTE). While performance is usually the primary concern in athletic contexts, when looking at general development the focus should be on maximizing positive training effects.
A primary consideration regarding musculoskeletal adaptation to resistance training is that muscle, bone, and tendon adapt at different rates to different stimuli throughout the youth developmental cycle. Common conditions seen in the youth population such as Sever’s apophysitis, Osgood-Schlatter, Sinding-Larsen-Johanssen, and avulsion fractures result from mismatches related to the loads placed upon an athlete. In particular, there seems to be a mismatch between rates of muscle and tendon adaptation, particularly among athletes participating in sports with a high volume of jumping.
According to Coffey and Hawley:
“…untrained individuals have a greater capacity to activate the molecular machinery in response to contractile activity, because any overload stimulus induces large perturbations to cellular homeostasis regardless of mode of exercise.”
However, being overly specialized can elicit maladaptation of the developing musculoskeletal system. We will now look at tissue-specific adaptations to loading among youth athletes.
Bone Health
According to Bonnet et al, the majority of bone mineral density (BMD) accumulation for the lifespan occurs during the pediatric years, with consolidation occurring from 18 to 35 years old. This is followed by an “involution” phase, in which BMD decreases over the remainder of the lifespan — although this can be mitigated with load-bearing exercise. Bone mineral density is typically measured using Dual X-ray absorptiometry (DXA) and reported as a Z-score, which is a statistical comparison to an age-matched “normal” control. The score represents the number of standard deviations a person’s bone density differs from their age-, sex-, and ethnicity-matched peers. For example, a Z-score of -1.0 represents a one-standard deviation decrease in BMD below that of their peers.

The type of exercise to which youth and adolescents are exposed influences adaptations in bone mineral density. Nichols et al performed a cross-sectional study of 161 female athletes categorized as either “repetitive” (swimming, running) or “multidirectional” athletes (everything else). Among this cohort of females (age 15.3 ± 1.3 years), those who participated in “repetitive” sports were three times more likely to have a low Z-score between -2.0 and -1.0 (29.4% vs. 9.7%), which falls in the range of clinical osteopenia. This suggests that even beyond early sports specialization, strictly participating in these “repetitive” loading sports can lead to skeletal maladaptation in adolescent females. The effect is also seen in males: Duplanty et al compared resistance-trained male runners to non-resistance trained and untrained peers, and found that:
“Resistance-trained runners had greater [bone mineral density] than nonresistance-trained runners and untrained peers. This difference did not seem to be modulated by biomarkers that contribute to bone formation or resorption, indicating that differences in [bone mineral density] are associated with habitual load-bearing exercise using external resistance.” (emphasis added)
Speckler et al conducted a systematic review on the role of exercise in pediatric bone and found that, compared to control groups not exposed to an exercise intervention, participation in exercise led to a 0.6% to 1.7% increase in annual bone accrual. These results were magnified in the cohort who were pre-pubertal, making the case that youth should exercise early and often to make gains in bone mineral density. These results have also been shown in adolescent females where a 15-month resistance training program not only increased strength by 40%, but increased bone mineral density at the femoral neck.
Sport-specific adaptation occurs in the upper extremities as well. In a 12-month longitudinal study by Ducher et al, pre/peri- and post-menarchal female tennis players underwent DXA scanning of their dominant and non-dominant arms. They found the increases in bone mineral content of 22.4% in dominant arms and 19.4% in non-dominant arms among the pre-/peri-menarchal group. In comparison, the slightly older post-menarchal group showed increases of 7.1% in the dominant arms and 4.9% in non-dominant arms. These results suggest that there are tissue-specific adaptations to loading patterns, and provides further confirmation that a large portion of bone accrual occurs in the earlier stages of adolescence.
Neuromuscular adaptation
It is evident that muscle incurs specific adaptations to mechanical loading, but the parameters of dosing (e.g., contraction mode & velocity, stimulus intensity, frequency, volume, etc.) heavily influence the specific adaptations generated in the trained population. There is likely no specific set/rep scheme that is “optimal” for initial adaptation among untrained individuals, as the novelty of the stimulus is typically sufficient to elicit changes in muscle in this population.
There does appear to be a discrepancy between the development of strength versus increases in muscle cross sectional area (CSA) in the earlier phases of training, particularly among youth athletes. Due to the constraints of measurement in youth, outcomes such as limb circumference and EMG “activation” tend to be used as proxies for neural and morphological changes even though they are not ideal measures. With this concession in mind, children do seem to show greater changes in voluntary activation post-training than adults (10-17% vs. 1.3-4.5%).
Other measurements of neurological adaptations include rate of force development (RFD), which describes the rate of increase in muscle force after contraction onset, and electromechanical delay (EMD), which describes the time lag between muscle activation and force production. Here, compared to adults children produce lower rates of force development and longer electromechanical delays. Some authors speculate this could contribute to the lower motor control typically seen in youth, as this result was mitigated in young, male gymnasts compared to their endurance-trained peers. The power development and motor control necessary to excel at gymnastics can still be improved, but this requires exposure to specific variables in training.
Muscle Morphological Changes
Morphological adaptations to heavy resistance training include increased cross-sectional area (CSA), changes in muscle architecture, and changes in fiber type, among others. It was originally thought that the majority of adaptation in prepubescent children had to be neurological due to their lower concentrations of endogenous anabolic hormones (e.g., testosterone) compared to adults. Narci et al demonstrated an initial increase in strength and hypertrophy in a nearly linear manner over 6 months of training in adult males, but this has not been demonstrated in youth and adolescents.
There is, however, a cross-sectional study from Kanehisa et al on the development of strength and muscle CSA through adolescence. While the cohort’s training history was unaccounted for (we assume they would likely be classified as “untrained”), they did find an association with age on both muscle CSA and strength outcomes, with marked increases occurring between the 10-12 year old cohort and the 13-15 year old cohort. This aligns with the optimum window from the Long-Term Athletic Development Model. The children in the cohort showing some of the biggest improvements were also in the middle school to early high school age range, long before resistance training is typically introduced into most training paradigms.
We must also consider the potential for even greater adaptations given a larger dose of training stimulus. In a different study Kanehisa et al followed seven of the top junior Olympic weightlifters in Japan for 18 months. Athletes were aged 15.5-17.1 years and had a training history of 2.5-4.1 years at the time of testing. The cohort showed specific adaptations of their quadriceps femoris muscle group and isometric torque increases that outpaced increases in muscle CSA. We have discussed at length the specific nature of strength adaptations, and It is likely that the demands of specific sports and training modalities elicit specific adaptations in youth and adolescent musculature, with those changes occurring rapidly through ages 13-15.
Changes in muscle architecture are also seen with resistance training. For example, eccentric-oriented exercise in the adult male population has demonstrated an ability to increase muscle fascicle length. Fascicles are naturally longer in adults than in youth and adolescents and vary by individual muscle. When normalizing for bone and muscle length differences between adult and youth populations, the differences become insignificant. Still, we do have some low-level evidence that training stimulus can elicit changes in muscle architecture among youth.
Finally, skeletal muscle loading increases muscles’ potential for repair by inducing proliferation of muscle stem cells (known as “satellite cells”) under both acute and chronic conditions.
Tendon
Whereas muscle is known to be a highly adaptive tissue, tendons typically take longer to adapt to loading. They contain a high concentration of extracellular matrix (ECM) with relatively few fibroblasts interspersed within. These features are advantageous for transmitting the tensile force generated by skeletal muscles to joints, but come at the cost of remodeling and repair being a much slower process. The half-life of tendon collagen has been reported at 10 times longer than that of skeletal muscle actin and myosin fibers. An often-cited study by Heinemeier looked at the presence of Carbon-14 radioisotopes in the Achilles tendons of individuals exposed to nuclear testing in the 1950’s, which aligns with the growth phase seen in bone development showing most tendon collagen accumulation occurs before the age of 17.
We also have abundant research related to tendon adaptation in the adult population, with research supporting the parameters that optimize tendon adaptation. There is also evidence on how tendon development changes with age. Kubo et al studied tendon compliance (a measure of stiffness) in the vastus lateralis muscles of young males (age 10.8 ± 0.9 years), slightly older youth males (age 14.8 ± 0.3 years) and young adult males (age 24.7 ± 1.6 years). They observed that tendon structures in younger males are more compliant (i.e., “stretchier”) than those in adults. The authors speculate this additional compliance could have a protective effect against athletic injuries, but this could be contingent upon sufficient development of the musculotendinous complex.
Much like bone, there may be a period of increased tendon development in youth. A 2-year longitudinal study performed by Mersmann et al studied muscle and tendon development in young, elite female volleyball players (age 16+/-1 year at entry into study). During this span, quadriceps muscle CSA and strength increased 6% and 13% respectively, while patellar tendon CSA increased 27%. These adaptations could be unique to volleyball athletes, but still seem to demonstrate an incongruence between the rates at which muscle and tendon adapt.
In a separate study Mersmann compared male and female volleyball athletes to age-matched controls. Irrespective of sex, muscle and tendon properties differed between athletes and controls. The volleyball athletes had increased muscle thickness and tendon stiffness, but had increased tendon strain as well. Cassel et al took this information one step further by studying youth athletes from different sports for Achilles and patellar tendon adaptation. A cohort of 500 youth athletes and 40 controls were broken into ball sports, combat sports, combined sports, cycling, and water sports, with each group demonstrating unique adaptations for the tendons. It must be mentioned that there was a large degree of heterogeneity in tendon thickness between each group, but overall the loading patterns and demands of each sport seemed to elicit specific, load-based adaptations to tendons, with the control cohort presenting with the smallest tendon thickness.
Tendons respond to specific types of loading that are often dichotomized into “low-load cyclic loading” and “high-magnitude loading”, with each eliciting unique responses in tendon development and repair. Low-load cyclic loading would be analogous to what is seen in repetitive activities (e.g., running) where tendon-related symptoms and diagnoses are common. From studies performed in adults, Arampatzis in 2007 and 2010 showed that low-load cyclic loading was insufficient to elicit meaningful Achilles tendon adaptations. As we have seen elsewhere, different sports seem to elicit specific adaptations to both tendon stiffness and cross sectional area.
Two meta-analyses by Bohm and Weisinger demonstrated that high-magnitude strain is necessary to elicit tendon adaptation in living tissue. High-magnitude strains are ideal for activating mechanotransduction pathways (e.g., via fibroblast deformation) that stimulate adaptive responses. One caveat is that plyometric exercise is typically included in the paradigm of high-magnitude strain, but research from Foure, Houghton, and Bohm would suggest that this is insufficient even when plyometric training is performed at relatively high intensities.
The work of Beyer and Kongsggaard examined the role of “heavy slow resistance” (HSR) training and eccentrics in the treatment of patients with symptomatic Achilles and patellar tendinopathy. Kongsgaard identified specific changes in collagen fibril morphology associated with heavy slow training. While this was a symptomatic population, it does lend support to the effects of heavy slow resistance training on tendon adaptation. The current dogmatic approach to treating tendinopathy involves the use of eccentric loading. However, the above-mentioned review by Bohm suggests that the type of muscle contraction (i.e., eccentric vs. concentric) is not as meaningful for tendon adaptation as the magnitude of tendon strain.
Specific to youth athletes, Mersmann et al analyzed the differences in muscle and tendon development as they relate to the development of tendinopathy. In order to maximize favorable tendon adaptations, they advocate for high-magnitude tendon strain using 5 sets of 4 repetitions at 85-90% 1RM with 3 seconds under tension for each repetition. This is the first prescriptive dosing to enter this article series, and may be better interpreted as an 8/9 RPE or even “hard”, since 5 sets of 4 repetitions at 85-90% of 1RM may be beyond what a well-trained individual could feasibly perform (particularly among specialized strength athletes). We will go much more into the relation of RPE, 1RM, and prescription later in the series.
Adiposity
The physiological adaptations to resistance training have also proven advantageous in the reduction of adiposity (i.e., body fat) in both normal weight and overweight children. In only 8-weeks of training McGuigan et al were able to decrease absolute body fat by 2.6% while increasing lean body mass by 5.3% in overweight and obese children. This cohort was also able to increase their 1RM squat by 74% over this period. This increase in strength is likely more a testament to how undertrained these kids were, but it remains notable that just two months of resistance training was able to elicit such a dramatic improvement.
McGuigan’s group took this experiment further by studying the effect of duration of training as well. Unsurprisingly, the longer the duration of training, the larger the effect on total fat mass, with the group training for 24 weeks showing an 8.1% decrease in total fat mass. Schranz et al conducted a meta-analysis on the effects and found very small to small effect sizes in favor of the implementation of resistance training on body composition, but the review also reported an influence of intervention type. Once again, proper dosing matters in order to elicit the desired effects, and operating under a homogeneous construct of “loading” likely is not nuanced enough to maximize benefit. Benson et al used a progressive high-intensity loading program of 2 sets of 8 repetitions across 11 exercises over 8 weeks and showed that both overweight and normal weight children demonstrated improvements in central, and whole-body adiposity.
In this piece we have laid out a limited review of the beneficial effects of resistance training across various tissues in youth athletes, including the nervous system, muscle, tendon, bone, and adipose tissue. As with all resistance training programs, the parameters of load and dosing have been shown to play a large role for specific adaptation. Beyond that, it is increasingly demonstrated that the duration of training is a major factor with longer programs demonstrating a larger effect. Meta-analyses by Behringer et al and Lesinski et al both found that longer programs elicited larger overall effects, further emphasizing the need for resistance training to be maintained as a long-term part of athletic development programs. In the next article we will discuss the roles of different training modalities in youth athletes.
